MORE THAN AN eCRF
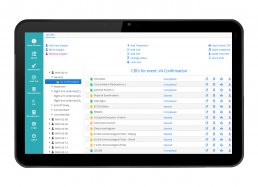
DataRiver has developed the “My Health eCRF” Web and Mobile Platform, for the management of clinical trials of Phase I – IV drugs, observational studies and trials on medical devices.
The My Health platform is validated (Computer System Validation) according to international standards and guidelines for clinical trials: ICH GCP, 21 CFR part 11 and IT security: OSSTMM, OWASP, NIST 800-115, ISO-IEC 27: 000 2016, ISO / IEC 27001: 2017, ISO / IEC 27002: 2013.
My Health eCRF allows to have a unified and complete view of information relating to patients enrolled in clinical trials, integrating the data collected through:
– a validated eCRF system for the management of Phase I – IV and observational clinical trials on drugs and clinical trials on medical devices
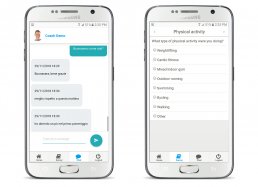
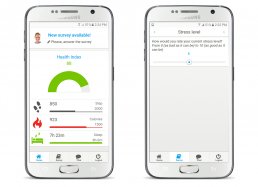
– app for administering patient questionnaires via smartphone and tablet
– wearable devices and sensors worn by patients to collect data on physical activity and rehabilitation performed
– medical devices for the collection of physiological parameters – the use of a direct doctor-patient communication channel through voice assistants and ChatBots
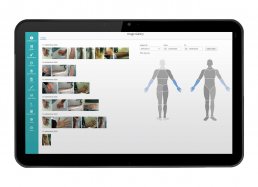
– the management of images and photos taken directly remotely by the patient
– the management of a televisit system that allows the medical staff to interact remotely with the patient
– the integration of clinical data collected in Electronic Health Record (EHR), clinical registries, Lab Database and eCRF systems
RELATED CASES
Solutions
RELATED CASES
Case studies