Electronic Trial Master File (eTMF)
Clinical Trial Document Management
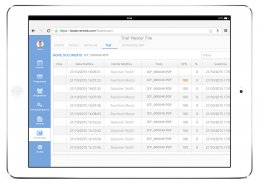
DataRiver has developed the Electronic Trial Master File system, available on PC and Tablet, that helps you organize and store documents, images and other digital content necessary for conducting clinical trials.
Features
Using this system helps you reduce the time required to manage documentation and activate of the clinical sites.
The system allows remote and real-time verification of documentation held directly at the clinical sites.
The system keeps you constantly updated on the status of each participating site through online documentation consultation
RELATED SOLUTIONS
The solutions
RELATED CASES
Case studies